Turning CO2 into Clean Fuel: A Breakthrough Faster, Cheaper and Poised to Reshape Our Fight Against Climate Change
In a major leap toward a sustainable energy future, scientists at the Korea Institute of Energy Research (KIER) have developed a groundbreaking catalyst that transforms carbon dioxide (CO2) into carbon monoxide (CO) is a key building block for eco-friendly fuels. This advancement could accelerate the global shift away from fossil fuels, reduce greenhouse gas emissions, and open new avenues for economic and social benefits worldwide.
A new era for carbon recycling. The team, led by Dr. Kee Young Koo, focused on improving the reverse water-gas shift (RWGS) reaction, a chemical process that converts CO2 and hydrogen into CO and water. Traditionally, this reaction requires extremely high temperatures and suffers from efficiency losses, making it expensive and technically challenging. The KIER researchers have designed a copper-based catalyst capable of operating at much lower temperatures, offering an economical and environmentally friendly pathway to carbon-neutral fuels.

This is not just a laboratory success. Efficiently converting CO2 into usable fuel components has immediate human and societal benefits. By turning a major greenhouse gas into a productive resource, this technology could help mitigate the health impacts of climate change, reduce air pollution, and contribute to energy security by creating domestically sourced fuels.
Innovation that balances science and society. Traditionally, RWGS reactions rely on nickel-based catalysts that require temperatures above 1,472°F and tend to degrade over time, decreasing efficiency. Low-temperature alternatives often produce unwanted byproducts like methane, which limits CO yields. Dr. Koo’s team tackled these problems by developing a copper-magnesium-iron mixed oxide catalyst that remains highly effective at just 752°F.

This catalyst does more than just improve efficiency, it addresses the social and economic dimensions of sustainable energy. Operating at lower temperatures reduces energy costs and infrastructure demands, making the technology more accessible to developing countries and smaller-scale industrial operators. Moreover, the use of abundant, inexpensive metals like copper, magnesium, and iron could make clean fuel production more equitable, avoiding reliance on expensive and rare materials like platinum.
How the catalyst works: The secret lies in a layered double hydroxide (LDH) structure, composed of thin metal sheets with water molecules and anions in between. By carefully adjusting the mix of metals, the team created a catalyst that prevents particle clumping and maintains thermal stability. Real-time testing showed that, unlike conventional copper catalysts that convert CO2 via intermediate formates, the new catalyst directly transforms CO2 into CO, minimizing wasteful byproducts.
The performance metrics are impressive: at 752°F, the catalyst achieved a CO yield of 33.4% and a formation rate of 223.7 micromoles per gram per second. It sustained continuous operation for 100 hours without significant loss of activity, outperforming even platinum-based catalysts in both speed and yield.
The global implications of this breakthrough are far-reaching. By providing a low-cost method to produce feedstocks for synthetic fuels, the technology aligns with international climate goals and carbon neutrality targets. Governments can integrate this approach into national decarbonization strategies, potentially reducing dependence on imported fossil fuels and strengthening energy sovereignty.
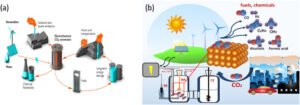
For emerging economies, the accessibility of such technology offers a chance to leapfrog traditional energy systems and adopt clean energy solutions rapidly. Investment in catalyst-based CO2 recycling could spur job creation, innovation hubs, and regional economic growth in green technology sectors.
The catalyst is particularly promising for e-fuels, which are made by combining green hydrogen with captured CO2. These fuels could decarbonize hard-to-abate sectors such as aviation and shipping, where conventional electrification is not feasible. KIER’s research, supported by its R&D initiative on sustainable aviation fuel (SAF) production, is a step toward making these ambitious targets achievable.
“The low-temperature CO2 hydrogenation catalyst technology is a breakthrough achievement that enables the efficient production of carbon monoxide using inexpensive and abundant metals” – said Dr. Kee Young Koo. “It is not just a scientific milestone but a pathway to practical, socially responsible energy solutions”.
Away from climate and energy policy, the catalyst could have tangible social benefits. Cleaner fuels reduce air pollution, lowering respiratory and cardiovascular health risks. Affordable synthetic fuel production could stabilize energy prices, reducing economic strain on households and industries. On a global scale, technologies like this support equitable climate solutions, enabling countries of all sizes and resources to participate in carbon reduction efforts.
![]()
The discovery opens the door to industrial-scale applications, and further innovations could enhance the efficiency, scalability, and sustainability of synthetic fuel production. As nations wrestle with the urgent challenge of climate change, this catalyst demonstrates that science, policy, and social well-being can align in creating a cleaner, more equitable energy future.
With a blend of technical innovation, economic accessibility, and social impact, this breakthrough shows how turning a greenhouse gas into fuel is not just chemistry, it is a transformative step toward reshaping the human relationship with energy and the environment.








